- info@medicalogy.com
- 0877 7555 4616
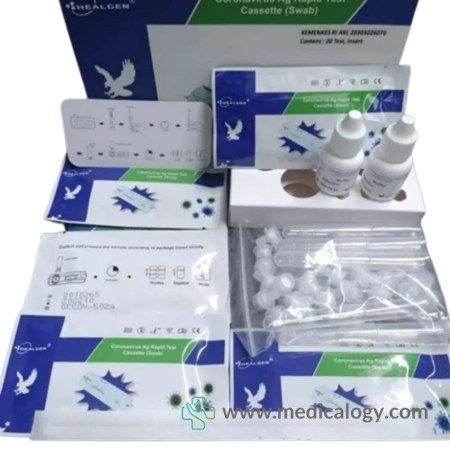
Healgen Covid-19 Ag Antigen Rapid Test Cassette Alat tes Coronavirus
Harga Healgen Covid-19 Ag Antigen Rapid Test Cassette Alat tes Coronavirus :
Deskripsi Healgen Covid-19 Ag Antigen Rapid Test Cassette Alat tes Coronavirus
The Rapid COVID-19 Antigen Test is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) swab from individuals who are suspected of COVID-19 by their healthcare provider. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections.
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. Currently, individuals infected by SARS-CoV-2 are the main source of transmission; asymptomatic carriers are also known as a source of transmission. Based on the current epidemiological information, the incubation period can last from 1 to 14 days, where the average time before symptoms occur is about 5 days. Symptoms include fever or chills, cough, shortness of breath, fatigue, muscle or body aches, headache, new loss of taste or smell, sore throat, runny nose, nausea or vomiting and diarrhea.
Clinical Evaluation
Positive Percent Agreement (PPA): 95.7%
Negative Percent Agreement (NPA): 99.6%
Overall Percent Agreement (OPA): 98.9%
Specimen: Nasopharyngeal (NP) Swab
Time to Results: 15 minutes
Shelf Life: 24 months from the date of manufacture
20 Tests/Kit
Review Healgen Covid-19 Ag Antigen Rapid Test Cassette Alat tes Coronavirus
Belum ada ulasan.